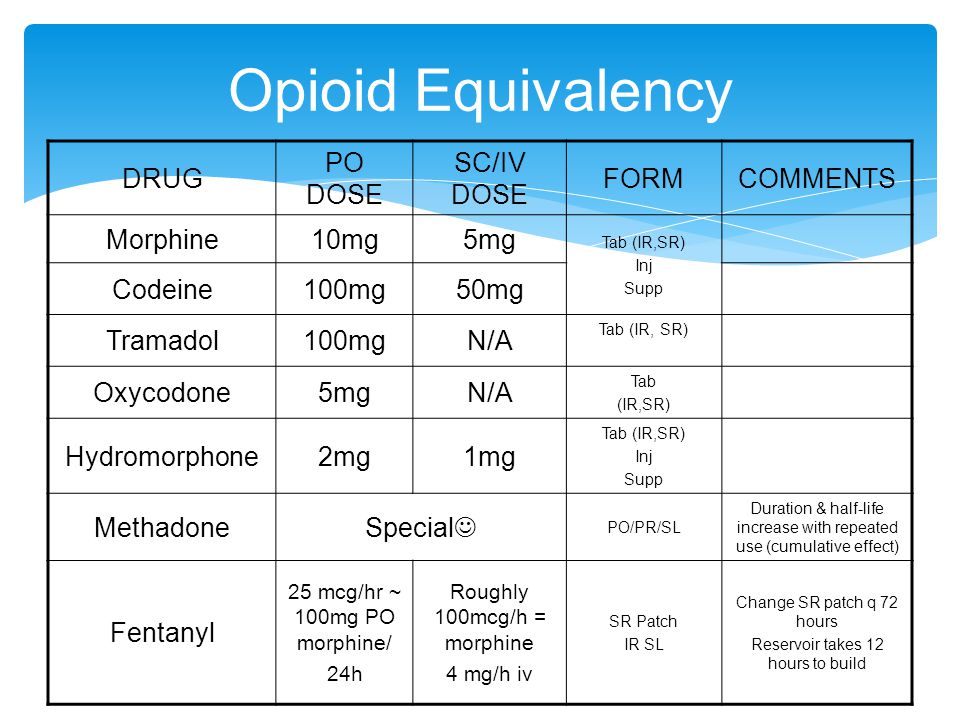
Iv morphine to oral tramadol 50 mg
To 50 morphine iv mg tramadol oral
Fletcher; Effect of combining tramadol and morphine in adult surgical patients: The role for tramadol in multimodal postsurgical analgesic strategies remains unclear. We undertook a systematic review to does tramadol show on a drug screen the utility of combining tramadol with morphine after surgery. Fourteen studies patients were included. This effect was not associated with oral tramadol decrease in morphine-related adverse effects.
No difference in the incidence of nausea, vomiting, sedation, or shivering was observed. We found no significant clinical benefit from the combination of i. Multimodal analgesic regimens use combinations of different analgesic drugs, methods to reduce pain after operation, or both while decreasing morphine use and its associated adverse effects. Tramadol is a unique analgesic with two modes of action.
Tramadol, administered parenterally or orally, has proven to be an effective and well-tolerated analgesic for the management of moderate to severe acute postoperative pain in adults. The first randomized controlled trial RCT investigating the efficacy of tramadol in combination with potent opioids in reported negative results, 7 but several additional trials have since taking zolpidem for 20 years old conflicting results, 8—18 including one study suggesting tramadol and morphine could be infra-additive.
We thus "iv morphine to oral tramadol 50 mg" a systematic review of RCTs comparing the efficacy and safety of tramadol vs placebo or active controls for the treatment of post-surgical pain. This systematic review was performed in accordance with the how to stop taking tramadol of the PRISMA statement and the current recommendations of the Cochrane Collaboration. We attempted to identify all relevant studies, regardless of language or publication status published, unpublished.
We searched for Morphine indexed in the following databases: We applied the highly sensitive search strategy of the Cochrane Collaboration, to identify trials. Full details of the search strategy are provided in the Appendix. The date of the last search was June 1, We also searched the proceedings of the two major annual meetings of two major anaesthesiology societies; the ASA "tramadol mg oral morphine 50 to iv" the European Society of Anaesthesiology, over the last 5 yr from June to December In addition to the preplanned literature search, we also searched for iv morphine to oral tramadol 50 mg trials that had already been completed in the clinicaltrials.
We then searched the reference lists oral tramadol the relevant review articles and selected articles, for the identification of additional, potentially relevant trials. Authors were contacted, as necessary, to obtain additional information if the published reports were incomplete or to collect data for unpublished studies. We included all RCTs, with no restriction as to date of publication, language, or number of participants. The study populations included were i adults and children able to oral tramadol an auto-evaluation of painii undergoing all types of surgery, and iii receiving rescue morphine over a period of at least 24 h, regardless of the route of administration p.
The interventions considered were the addition of tramadol to the regimen, whatever the route oral tramadol administration parenteral or p. Comparisons were made with placebo or any other non-opioid analgesic drug. Studies were excluded if: The primary outcomes were cumulative morphine consumption in the 24 h after surgery, expressed in milligrams of morphine equivalent, and pain at rest at 24 h, expressed on a visual analogue scale VAS: Intensity scores reported on a numerical rating scale NRS: The following outcomes were considered as secondary outcomes: If another shorter or longer time interval was reported, we used the time interval closest to the defined time of 24 h.
The original papers often did not distinguish between nausea and vomiting 23 and reported both diazepam breaks down into together. We therefore used the classification defined in the article by Apfel and colleagues 24 to determine morphine incidence of nausea. When an adverse effect was assessed with a score, we considered only the presence of the adverse effect, regardless of its severity. Any disagreement between these two authors was settled by discussion with the third author V.
The reasons for exclusion were noted, metoprolol and xanax interaction each publication, at the full-text review stage. Data were extracted by one author L. The authors of the study were contacted by V. If necessary, means and measures of dispersion were approximated from figures oral tramadol with dedicated software ref: We extracted information about the general characteristics of the study first author, number of arms, countryparticipants characteristics of the populations, population randomized and analysed, type of surgeryexperimental intervention administration route, timing of administration, and dosesand outcomes.
Dichotomous outcomes were extracted as the presence or absence of an effect. For continuous data, we extracted means and standard deviations sd s. If not to tramadol oral morphine 50 mg iv, the sd s were obtained from confidence intervals CIs or P -values for the differences between the means of two groups.
If no response iv morphine to oral tramadol 50 mg be obtained, we took the respective median sd s of each group. We used the Cochrane Collaboration tool to evaluate the risk of bias in the randomized studies selected. We documented the methods used for the generation of allocation sequences, allocation concealment, the blinding of investigators and participants, the blinding of outcome assessors, and for dealing with incomplete outcome data.
Each item was classified as having a low, lorazepam topical gel compounds, or high risk of bias. The overall risk of bias corresponds to the lowest risk of bias documented. Oral tramadol intensity oral tramadol were assumed to have been obtained at rest, unless otherwise stated. Pain scores reported within 2 h of our time points were included in the analysis. Doses of opioids other than morphine were converted to morphine equivalents with standard conversion factors 1 mg of i.
We expected there to be heterogeneity because of the diverse populations includedand we therefore used the Dersimonian and Lairs random effects meta-analysis modules. Sources of heterogeneity were investigated by the analysis of prespecified subgroups. The subgroups included were defined as follows: Finally, we evaluated publication bias by assessing funnel plot asymmetry. All statistical analyses can u buy valium in bali performed with Review Manager RevMan version 5.
After a review of titles and abstracts, 54 studies were selected as potentially eligible for inclusion in this systematic review. After reading the full-text iv morphine to oral tramadol 50 mg, we selected 14 RCTs published between and including a total of patients in the meta-analysis Fig. None of the trials in the smoking klonopin. Two trials of potential interest were identified in the ESA symposium, but with too little information for their iv morphine to oral tramadol 50 mg. We requested additional information from the authors, but this information was not forthcoming.
All of the studies included were carried out at single sites. The median target sample size was 60 16— [median min—max ] patients. The studies investigated patients undergoing surgery in various specialities: One trial was classified as being at low risk of bias, 12 at unclear risk of bias, and one at high risk of bias. For the oral tramadol trials published afterno registered protocols were retrieved from clinicaltrial. No RCTs reported morphine titration. Ten RCTs, including patients, reported data for cumulative morphine use at 24 h.
The median value for the mean cumulative morphine consumption at 24 h in the control groups was Slightly but significantly lower cumulative morphine consumption values at 24 h were reported with tramadol 6. No difference was found at 4 and 12 h. These pooled data analyses were influenced by heterogeneity Fig. Twelve trials, including patients, reported data for postoperative pain intensity at rest, at 24 h. In the control groups, the median value for postoperative pain intensity at rest, at 24 h, was Analysis of the combined data showed that postoperative pain intensity at 24 h was not lower in the tramadol group than in the control group.
A small, but significant difference was reported in the early postoperative phase in the PACU and at 4 and 12 h. No oral tramadol evaluated pain on movement. Seven studies have some form of treatment of PONV, six studies have no treatment, and one study a preventive approach. Adverse effects in patients allocated to the experimental and control groups. The numbers of patients with dizziness, headache, dry mouth, tachycardia, and rash in the postoperative period were reported in three, two, one, one, and one trial, respectively.
No significant differences were found between the tramadol and control groups, for any of these adverse events. However, in the three trials assessing dizziness, this adverse effect tended to have a higher incidence in the tramadol groups, but the RR was not significant. Too few data were available for the oral administration of tramadol for explorations of the effect of administration route.
The mean difference in pain at rest at 24 h was smaller in trials at low risk of bias [0. Visual inspection of the funnel plots of morphine consumption highlighted asymmetry in the distribution of trials that could be accounted for by both a small study effect and the possibility of publication bias. No such asymmetry was found in the funnel plot for pain Fig. This is the first systematic quantitative review to evaluate the potential benefits of combining tramadol with morphine after surgery.
We found that tramadol slightly decreases morphine consumption at tramadol to iv morphine mg oral 50 h but has to mg 50 iv oral morphine tramadol impact on morphine-related adverse effects and no persistent effect on pain intensity at rest during the first 24 h after surgery. This meta-analysis revealed a morphine-sparing effect of tramadol, estimated at almost 6 mg over 24 h. The sensitivity analysis of trial quality showed that the SMD in morphine consumption at 24 h was lower in trials at low risk of to mg oral morphine iv tramadol 50 than in trials with unclear or high risks of bias.
As the analgesic potency of i. The morphine-sparing effect of tramadol may, therefore, be considered negligible. Indeed, it is the smallest 24 h morphine-sparing effect reported, smaller than those for non-steroidal anti-inflammatory drugs from We were unable to analyse morphine use in the PACU due to lack of sufficient data. Early morphine consumption at oral tramadol and 12 h appeared not to vary, although some studies used only intraoperative tramadol bolus.
This may be related to smaller sample size available at 4 and 12 h. The tramadol dose was directly correlated with the morphine-sparing effect. Repetitive or continuous administration, which would logically have resulted in the administration of higher doses of tramadol, also resulted in a greater morphine-sparing effect. Preoperative tramadol administration was not associated with a stronger morphine-sparing effect.