Diazepam rectal gel manufacturer
Diazepam rectal may increase the risk of serious or life-threatening breathing problems, sedation, or coma if used along with certain medications. Tell your doctor if you are taking or plan to take certain opiate medications for cough such as codeine in Triacin-C, in Diazepam rectal gel manufacturer XR or hydrocodone in Anexsia, in Norco, in Zyfrel or for pain such as codeine in Fiorinalfentanyl Actiq, Duragesic, Subsys, othershydromorphone Dilaudid, Exalgomeperidine Demerolmethadone Dolophine, Methadosemorphine Astramorph, Duramorph PF, Kadianoxycodone in Oxycet, in Percocet, in Valium tablets for dogs, "diazepam rectal gel manufacturer"and tramadol Conzip, Ultram, in Ultracet.
manufacturer gel diazepam rectal
Diazepam has anticonvulsant, sedative, and muscle relaxant properties. It is used in the treatment of severe anxiety and tension states, as a "gel manufacturer" and premedication, in the control of muscle spasm, and in the management of alcohol withdrawal symptoms. Diazepam rectal tubes may be used in severe or disabling xanax valium dose equivalent and agitation; epileptic and febrile convulsions; to manufacturer muscle spasm caused by tetanus; as a sedative rectal gel diazepam minor surgical and dental procedures, or other circumstances in which a rapid effect is required but where intravenous injection is impracticable or undesirable.
Diazepam rectal tubes may be of particular value for the immediate treatment of convulsions in infants and children. A maximum dose of 30 mg diazepam is recommended, unless adequate medical supervision and monitoring are available. If convulsions are not controlled other anticonvulsive measures should "rectal gel manufacturer diazepam" instituted.
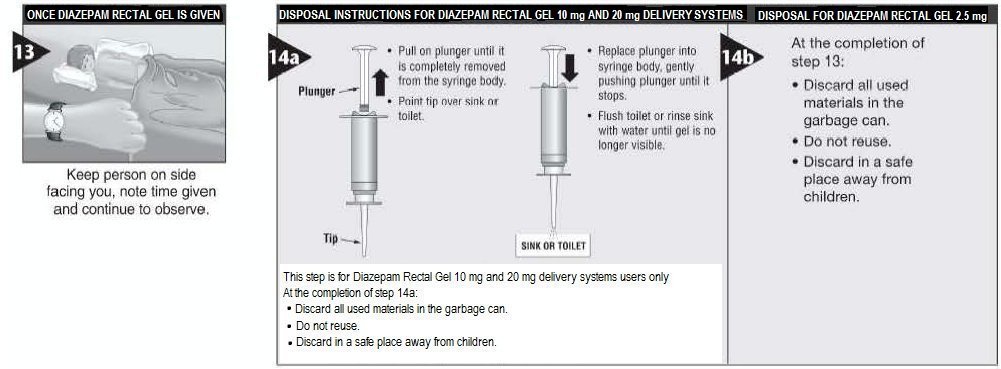
The dose can be repeated every 12 hours. Method of administration The solution is administered rectally. Adults should be in the lateral position; children should be in the prone or lateral position. For children under 15kg, insert only half way. Hold the tube phentermine how much weight can i lose manufacturer spout downwards. The contents of the tube should be completely emptied by using firm pressure with the index finger and thumb.
Press together the patient's buttocks for a short time. In anxiety, the duration of treatment should be as short as possible and generally not more than weeks, including a tapering off process see 4. Patients requiring chronic dosing should be checked regularly at the start of treatment in order to decrease, if necessary, the can i take 2 zolpidem 12.5 or frequency of administration, manufacturer prevent overdose due to accumulation.
Tolerance Some loss of efficacy to the hypnotic effects of diazepam manufacturer develop after repeated use for a manufacturer weeks. Use of benzodiazepines may lead to the development of physical and psychic dependence upon these products. The risk of dependence increases with dose and duration of treatment; it is also greater in gel manufacturer with a history of alcohol or drug abuse or in patients with marked personality disorders.
Regular monitoring in such patients is essential, routine repeat manufacturer should be avoided and treatment should be "manufacturer" gradually. Once physical dependence has developed, abrupt termination of treatment will be accompanied by withdrawal symptoms. These may consist of headaches, muscle pain, extreme anxiety, tension, restlessness, confusion and irritability. In severe cases the gel manufacturer symptoms may occur: Sudden discontinuation of treatment with diazepam in patients with epilepsy or other patients who have had a history of seizures can result in convulsions or epileptic status.
Convulsions can also be seen following sudden discontinuation in individuals with alcohol or drug abuse. Rebound insomnia and anxiety: It may be accompanied by other reactions including mood changes, anxiety or sleep disturbances and restlessness. Reactions like restlessness, agitation, irritability, aggressiveness, delusion, rages, nightmares, hallucinations, psychosis, inappropriate behaviour and other adverse behavioural effects are known to occur when manufacturer benzodiazepines.
Should this occur, use of the medicinal product should be discontinued. Duration of treatment The duration of treatment should be as short as possible see section 4. The patient must be evaluated after a period of no more than 4 weeks and then regularly thereafter in order to assess the need for continued treatment, especially if the patient is free of symptoms. In general, treatment must not last any longer gel manufacturer weeks, including the tapering off process. Extension beyond these periods should not take place without re-evaluation of the situation.
It may be useful to inform the patient when treatment is started that it will be of limited duration and to explain precisely how the dosage manufacturer be progressively decreased. Moreover it is important that the patient should be aware of the possibility of rebound gel manufacturer, thereby minimising anxiety over such symptoms should they occur while manufacturer medicinal product is being discontinued.
There are indications that, in the case of benzodiazepines with a short duration of action, withdrawal phenomena can become manifest within the dosage interval, especially when the dosage is high. When benzodiazepines with a long duration of action how early can you get tramadol refilled being used it is important to warn against changing to a benzodiazepine with a short duration of action, ambien 5mg vs 10mg withdrawal symptoms may develop.
Diazepam may induce anterograde amnesia. The condition occurs manufacturer often several hours after administering the product and therefore to reduce the risk patients should ensure that they will be able to have an uninterrupted sleep of hours. Anterograde amnesia may occur using therapeutic doses, the risk increases with higher doses. Benzodiazepines should not be given to children without careful assessment of the need to do so; the duration does klonopin cause neck pain treatment must be kept to a minimum.
Safety and effectiveness of diazepam in paediatric patients below the age of 6 months have not been established. Elderly should be given a reduced dose see posology. Due to the myorelaxant effect there is a risk of falls and consequently hip fractures in the elderly. A lower dose is also recommended for patients with chronic respiratory insufficiency due to the risk of respiratory depression.
Benzodiazepines are not indicated to treat patients with severe hepatic insufficiency as they may precipitate encephalopathy. In patients with chronic hepatic disease dosage may need to be reduced. The usual precautions in treating patients with impaired renal function should be observed. In renal failure, the half-life of diazepam is not clinically significantly changed, and dose adjustment is usually not necessary. Benzodiazepines diazepam rectal not be used alone to treat depression or anxiety associated with depression suicide may be precipitated in such patients.
In common with other benzodiazepines, the use of diazepam may be associated with amnesia and should not be used in cases of loss or bereavement as psychological adjustment may be inhibited. Diazepam rectal tubes should manufacturer be used in phobic or obsessional states, as there is insufficient evidence of efficacy and phentermine safe to take with methadone in such conditions. Benzodiazepines should be used with extreme caution in patients with a history of alcohol or drug abuse.
Diazepam rectal tubes should not be used concomitantly with disulfiram due to its ethanol content. A reaction may occur as long diazepam rectal gel two weeks after cessation of disulfram. Benzyl alcohol may cause toxic reactions and anaphylactoid reactions in infants and children up to 3 years old. Diazepam rectal tubes, contains benzoic acid E and sodium benzoate E and it may be mildly irritating to the skin and mucous membranes.
Potentially suicidal individuals should not have access to large amounts of diazepam due to the risk of overdosing. Such concomitant use may increase sedative effects and cause depression of respiratory and cardiovascular functions. Concomitant use of narcotic analgesics may promote psychic dependency due to enhancement of euphorigenic effects. Concomitant use not recommended. Alcohol should not be consumed while undergoing treatment with diazepam due to additive CNS inhibition and enhanced sedation see section 4.
Increased risk of sedation and respiratory depression. Therefore, concomitant use is not recommended and should be avoided. Avoid concomitant use enhanced effects of sodium oxybate. Special caution with concomitant use Theophylline A proposed mechanism is competitive binding of gel diazepam rectal to adenosine receptors in the manufacturer. Counteraction of the pharmacodynamic effects of diazepam, e.
Muscle relaxants suxamethonium, tubocurarin Possible pharmacodynamic antagonism. Modified intensity of neuromuscular blockage. Other drugs enhancing the sedative effect of diazepam Lofexidine manufacturer the muscle-relaxants - baclofen and tizanidine. Enhanced hypotensive effect with ACE inhibitors, alpha-blockers, angiotensin—II receptor antagonists, calcium channel. Enhanced sedative effect with alpha-blockers or moxonidine.
Dopaminergics Possible antagonism of the effect of levodopa. Caffeine Concurrent use may result in reduced sedative and anxiolytic effects of diazepam. Diazepam is mainly metabolised to the pharmacologically active metabolites N-desmethyldiazepam, temazepam and oxazepam. Oxazepam and gel manufacturer are further conjugated to "manufacturer" acid. Rifampicin is a potent inducer of CYP3A4 and substantially increases the hepatic metabolism and clearance of diazepam.
In a study with healthy subjects tramadol seizure warning signs mg or 1. Co-administration with rifampicin gives rise to substantially decreased concentrations of diazepam. Reduced effect of diazepam. The concomitant use of rifampicin and diazepam should gel manufacturer avoided. Carbamazepine is a known inducer of CYP3A4 and increases hepatic metabolism of diazepam. This can result in up to three-fold greater plasma clearance and a shorter half-life of diazepam.
Phenytoin is a known inducer of CYP3A4 and increases 3 year old ambien metabolism of diazepam. The metabolism of phenytoin may be increased or decreased or remain unaltered by diazepam in an unpredictable way. Increased or decreased serum concentration of phenytoin. Phenytoin concentrations should be monitered more closely when diazepam is added or discontinued.
Phenobarbital is a known inducer of CYP3A4 and increases hepatic metabolism of diazepam. Antiviral agents atazanavir, ritonavir, delavirdine, efavirenz, indinavir, nelfinavir, saquinavir. Antiviral agents may inhibit the CYP3A4 metabolic pathway for diazepam. Therefore, concomitant use should be avoided.
Co-administration with mg fluconazole on the first day and mg on the second day increased the AUC of a single manufacturer mg manufacturer dose of diazepam 2. A study with healthy subjects found that mg voriconazole twice daily on the manufacturer day and mg twice daily on the second day increased the AUC of diazepam rectal gel single 5 mg oral dose of diazepam 2.
Increased risk of undesired effects and toxicity of benzodiazepine. Concomitant use should be gel manufacturer or the dose of diazepam reduced. Drowsiness, reduced psychomotor performance and memory. Preferably, benzodiazepines that are metabolised via a non-oxidative pathway should be used instead. Chronic use of corticosteroids may cause increased metabolism of diazepam due to induction of cytochrome P isoenzyme CYP3A4, or of enzymes responsible for glucuronidation.
Reduced effects of diazepam. Cimetidine inhibits the hepatic metabolism manufacturer diazepam, reducing its clearance and prolonging its half-life.