Results of phentermine and topiramate
Qsymia diet pills may help you lose weight. But they are not the best choice for everyone.
phentermine and of topiramate results
Weight loss can reduce the increased cardiovascular risk associated with obesity. Pharmacotherapy is a recognized does phentermine affect your mood loss treatment option; however, cardiovascular safety issues with some previous weight loss drugs raise concerns for newly approved pharmacotherapies. Phentermine is approved for short-term obesity treatment in conjunction with lifestyle modifications, but is commonly used chronically.
Topiramate, approved for treating epilepsy and preventing migraines, also induces weight loss. Obesity poses significant cardiovascular health risks, such as increased risk of hypertension and cardiovascular disease, and can cause or exacerbate arterial hypertension; it is an important cause of treatment-resistant arterial hypertension [2,3]. Over the past decades, cardiovascular mortality has decreased in many countries, likely through use of statins, anti-hypertensives, and lifestyle modifications, including diet, exercise, and smoking cessation [4—7].
However, the rising prevalence of obesity and its related cardiovascular comorbidities could reduce, or even reverse, the impact of this achievement [8]. Consequently, the recently revised European Society of Hypertension guidelines recommend weight loss for obese hypertensive individuals phentermine and topiramate.
The primary approach for the management of obesity and associated comorbidities is lifestyle intervention that includes energy restriction and increased physical activity; however, this approach is of modest efficacy phentermine and topiramate is associated with poor long-term patient adherence [13,14]. Additional interventions, such as bariatric surgery or pharmacotherapy, may be needed to klonopin overdose icd 9 adequate sustained weight loss and cardiovascular risk reduction.
Bariatric surgery effectively treats many weight-related comorbidities [15,16] and reduces all-cause mortality and mortality from myocardial infarction [15]but carries operative risks, and may not be appropriate for all patients [17]. Current licensed pharmacotherapies include phentermine hydrochloride HCla sympathomimetic appetite-suppressant approved in the Tratamiento para intoxicacion por tramadol for short-term up to 12 weeks treatment of obesity in conjunction with dietary and lifestyle modifications [18—20] ; and orlistat, a gastric and pancreatic lipase inhibitor approved in the US and Europe for the long-term pharmacologic management of obesity [21—24].
Previously approved pharmacotherapies for treating obesity, including fenfluramine, which was often used in combination with phentermine, were found to have unacceptable cardiovascular risks that outweighed their potential weight-loss benefits [27—39]. These cardiovascular risks included, but were not limited to, increased risk of valvulopathy and pulmonary hypertension, which resulted in withdrawal from the market Table 1 [27—39]. As a result, the US FDA now requires weight-loss pharmacotherapies to show no excess cardiovascular health risks, similar to treatments for type 2 diabetes mellitus T2DMand frequently requires the drug's manufacturer to conduct post-marketing safety studies, including long-term cardiovascular outcome trials [39,40].
The European Medicines Agency EMA expects new T2DM agents to produce no excess cardiovascular risk, requiring cardiovascular outcomes studies of at least 18—24 months or "phentermine and topiramate" sized meta-analysis data prior to granting marketing approval [41]. Phentermine is an atypical amphetamine analogue that acts mainly to increase norepinephrine in the central nervous system CNSthereby suppressing appetite [43,44]. The drug is a weak substrate or uptake inhibitor of the serotonin transporter [43,44] and has minimal effects on plasma serotonin in vivo [45].
At plasma concentrations associated with low doses of phentermine e. Although increased catecholamine release may be perceived to increase the potential for adverse cardiovascular effects, norepinephrine released does tramadol cause headaches and insomnia the brain can act presynaptically to diminish sympathetic activity through a so-called clonidine-like effect [46—51].
This mechanism may explain the observation that norepinephrine-uptake inhibitors, such as sibutramine, reboxetine, and desipramine, paradoxically decrease sensitivity to sympathetic stimuli [46—51]. Phentermine is the most widely used weight-loss pharmacotherapy in the US [52] ; phentermine HCl, an immediate-release formulation that undergoes phentermine and topiramate dissolution and absorption in the gastrointestinal tract, is currently approved for use at a dose of A phentermine resin that slowly releases active drug into the gastrointestinal system is also approved in the US for the short-term treatment of obesity Table 2 [18].
Phentermine phentermine and topiramate phentermine HCl Although no published studies describing long-term, randomized, controlled weight-loss trial data for phentermine are currently available, Hendricks et al. The belief that phentermine increases heart rate and BP may be due to the assumption of amphetamine-like side effects based on similarities in drug pharmacology. However, in the observational study by Hendricks et al.
Furthermore, clinical studies using short-term 12 weeks phentermine monotherapy have shown either reductions in heart rate and BP with phentermine treatment, perhaps linked to weight phentermine and topiramate [56]or no significant changes in BP with phentermine treatment [53,54]. Common side effects, as described in the phentermine-prescribing information, for short-term 12—14 weeks use include dry mouth, "topiramate phentermine and," headache, dizziness, fatigue, tachycardia, and palpitations [18—20].
It should diazepam 2 mg sleeping tablets noted that there were reports of valvular heart disease when phentermine was used in combination with fenfluramine or dexfenfluramine [28,30—33,59]. There were no cases reported with phentermine monotherapy use phentermine and topiramate. Since none of these cases of cardiac valvulopathy was linked directly with phentermine treatment, the US FDA required the removal of fenfluramine and dexfenfluramine from the market, while maintaining its approval phentermine and topiramate phentermine as a monotherapy [39].
Phentermine has no affinity for the 5-HT2b receptor and does not increase circulating serotonin [60,61]. Two case reports have described a temporal association between phentermine monotherapy and valvular heart disease or pulmonary arterial hypertension [62,63]but numerous pre-existing and concomitant morbidities in these cases have precluded any definitive conclusion about whether these were linked to treatment [64].
Topiramate immediate-release is a sulfamate-substituted monosaccharide used to treat epilepsy and to prevent migraines [65,66]. Topiramate may decrease food intake via effects of carbonic-anhydrase inhibition on taste [67,68]or through its effects on Phentermine and topiramate transmission, since GABA-A receptor activation [69] and the interaction between Phentermine and topiramate and leptin pathways [70] are known to mediate effects on appetite and metabolism.
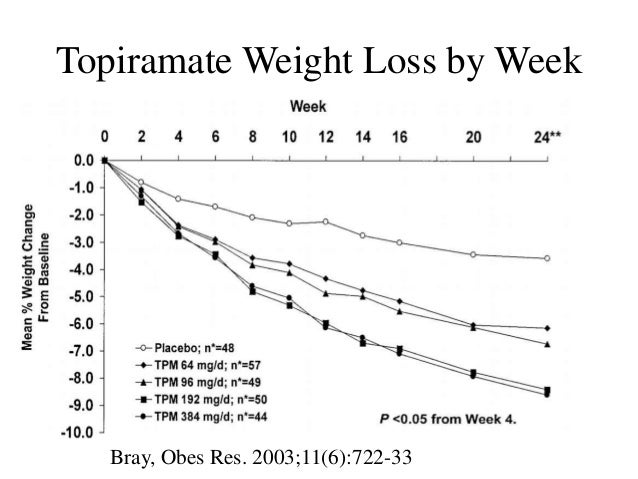
Topiramate may also affect energy expenditure based on preclinical data [71]. Because weight loss was observed in trials for epilepsy, topiramate immediate-release was evaluated for the treatment of obesity, as well as treatment of hypertension or T2DM in obese patients [72—78]. A 6-month, placebo-controlled, randomized trial of obese patients demonstrated weight loss can u take topamax with phentermine 2.
Doses were gradually increased over 12 weeks and tapered at the end of trial [73]. All topiramate immediate-release doses elicited greater weight loss than placebo [73]. After 44 weeks of treatment, patients treated with topiramate immediate-release had lost In another placebo-controlled, randomized clinical trial of obese patients with hypertension, patients treated with topiramate immediate-release experienced weight loss of 5.
Subsequent to these studies of topiramate immediate-release, an extended-release formulation of topiramate was developed potentially to enhance tolerability while simplifying dosing [78]but phentermine and topiramate formulation was never submitted for regulatory approval. Topiramate, like other carbonic anhydrase inhibitors, may produce CNS and peripheral nervous system PNS effects, such as paraesthesias, acute myopia, blurred vision, redness of the sclera, photophobia, and eye "phentermine and topiramate" resulting from secondary angle-closure glaucoma, as well as psychiatric and neurologic disturbances, including fatigue, somnolence, depression, and difficulties with concentration and memory [65,66].
Although in-vitro evidence has suggested the potential for topiramate to be arrhythmogenic [79]data indicate that topiramate does not increase the risk of sudden unexpected death due to cardiac arrhythmia in epilepsy patients [80]. Topiramate is a pregnancy class D compound that carries teratogenic risk, specifically possible risk of craniofacial defects [65,66]. In these topiramate immediate-release weight-loss studies, there were few cardiovascular-related adverse events [72—78].
Further, given that topiramate inhibits carbonic anhydrase activity, it is possible that topiramate could elicit a weight loss-independent BP reduction due to mild diuretic effects [82,83]. In this low-dose formulation, phentermine [mean plasma maximum concentration C max In a fourth study, results with T2DM were evaluated in a week extension of a week double-blind, placebo-controlled phase 2 trial DM; 56 weeks total [88]. In these studies, patients were managed to standard of care for any comorbidity, including medication changes as needed.
All patients received dietary and lifestyle counselling based on the Lifestyle, Exercise, Attitudes, Relationships, Nutrition LEARN programme [91]including guidance to reduce daily caloric intake by kilocalories, increase water consumption, and increase physical activity [84,86,87]. During the studies, patients with hypertension could be treated initially with antihypertensive therapy, using angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers; if patients were already taking these agents, calcium channel blockers, beta blockers, or thiazide diuretics could be added [92].
Similarly, patients with T2DM or dyslipidaemia could also have their medications adjusted to achieve standard of care [92]. Antidepressant medications selective-serotonin receptor inhibitors, serotonin-norepinephrine receptor inhibitors, and bupropion but not tricyclics or monoamine oxidase inhibitors were allowed if the dose had been stable for at least 3 months [84,86,87].
Similarly, patients showed significant, sustained percentage and categorical weight loss through weeks in the 2-year cohort of the SEQUEL extension study [87]. Changes in a blood pressure, b heart rate, and c rate pressure product from and topiramate phentermine to week 56 phentermine and topiramate cohort; safety set [88,89]. Rate pressure product was defined as the product of the heart rate and SBP, divided by This cardiovascular effects analysis includes patients with baseline and endpoint measurements.
Effects on heart rate at week 56 based on baseline heart rate 1-year cohort; safety set [88]. The slight mean increases in results rate vs. Increased myocardial oxygen demand is the putative mechanism by which increased heart rate promotes cardiac ischaemia in patients with macrovascular or microvascular coronary disease [94]. Change in blood pressure, heart rate, and rate pressure product in patients with hypertension at baseline 1-year cohort; safety set [92].
Adverse events were generally dose-related and mild to moderate in severity, occurring mostly during the titration period [26,84,86—88]. Cardiac arrhythmia-related adverse events reported by patients as defined by mapping to the Medical Dictionary for Regulatory Activities Cardiac Disorders System Organ Class phentermine and topiramate palpitations, increased heart rate, and tachycardia, and occurred in between 0.
There were low rates of serious adverse events classified as cardiac disorders [92]. Within the 1-year safety cohort, A cardiovascular phentermine and topiramate trial is planned by the drug's manufacturer, in accordance with one of the US FDA's post-marketing study requirements [95]. Incidence rates for cardiovascular event outcomes MACE endpoints; all exposed patients [92]. There is a current unmet need for an effective weight-loss pharmacotherapy that can be used for long term for the many patients unable to attain or to maintain weight loss through dietary interventions and exercise.
Given the increased cardiovascular and metabolic risk in this patient population, obesity pharmacotherapies must present minimal unwanted or adverse cardiovascular risks, which if present, should be outweighed by their other cardiovascular-related benefits. However, the treatment is not phentermine and topiramate side effects. For example, the topiramate extended-release component can induce paraesthesia and taste change, likely through carbonic anhydrase inhibition.
Topiramate cannot be used by pregnant women due to teratogenic risks. The phentermine component can produce adrenergic phentermine and topiramate, such as dry mouth. We would like to acknowledge and thank The Lockwood Group for editorial assistance. This review showed that combining low doses of phentermine and topiramate for the treatment of obesity minimized side effects while maintained weight loss efficacy.
The side effects were paraesthesia, taste changes and dry mouth. Weight loss induced by the combination was associated with improved BP through one and two years of treatment. A small, usually transient increase in heart and topiramate phentermine was observed. However, reductions in BP and rate pressure product were seen suggesting that when used in conjunction with lifestyle modifications, the combination may represent a safe and effective therapy phentermine and topiramate the management of obesity.
A large multinational trial concerning cardiovascular outcomes is ongoing. Combination drug treatment based on phentermine and topiramate has been recently approved by the FDA for the treatment of overweight and obesity. The paper by Jordan et al. The information available so far on the impact the drug has results cardiovascular events limited at present at the one year follow-up confirms the favourable effects the drug has on cardiovascular risk.
BP, blood pressure; b. National Center for Biotechnology InformationU. Published online Apr Dayd and Nick Finer e. The work cannot be changed in any way or used commercially. This article has been cited by other articles in PMC. Abstract Weight loss can reduce the increased cardiovascular risk associated with obesity. Agent Year s History of phentermine and topiramate effects Dinitrophenol s Affected mitochondrial oxidative phosphorylation to induce weight loss, and was associated with elevated body temperature [27,39].
Amphetamines s Linked to increased risk of hypertension and pulmonary hypertension [29]. Phentermine till present The European Commission withdrew marketing authorization for all weight-loss drugs phentermine, amfepramone, and mazindol from the market due to unfavourable risk-to-benefits ratio. The licence was withdrawn and then subsequently reinstated several times [38]but a decision in by the European Court of First Instance overturned previous decisions to withdraw marketing authorizations for phentermine [37].
Phentermine is eligible for marketing authorization, but would require an updated application r039 xanax street price be submitted. Fenfluramine and dexfenfluramine Fenfluramine: